PDMP ADMINISTRATION
Nationally, PDMPs are operated by various state agencies including health, board of pharmacy, law enforcement, etc. Funding for PDMPs is generally through state general funds, but may be supplemented with grants and dedicated state funds. Understanding how and when PDMPs were established and how they evolved provides a better understanding of PDMPs today. PDMP Administrators, stakeholders and the public will be interested in the history of PDMPs and other relevant PDMP information.
BJA Harold Rogers PDMP Grant Recipients
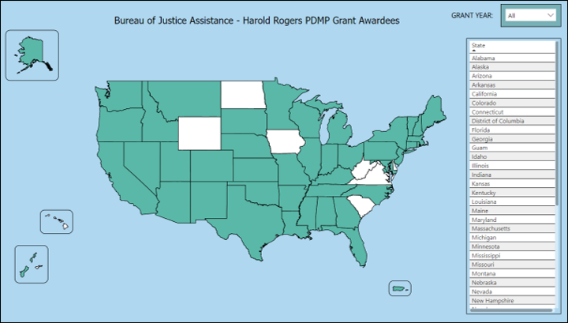
The Bureau of Justice Assistance (BJA) Harold Rogers Prescription Drug Monitoring Program (PDMP) Grant enhances the capacity of regulatory and law enforcement agencies and public health officials to collect and analyze controlled substance prescription data and other scheduled chemical products through a centralized database administered by an authorized agency. The goal of the Harold Rogers PDMP Grant is to support state and local governments in detecting and preventing the diversion and misuse of pharmaceutically controlled substances such as opioids and other prescription drugs. Grant funds may be used to support a variety of PDMP implementation or enhancement activities that encourage the use of PDMPs to improve clinical decision making and prevent the misuse and diversion of controlled substances.
TTAC VisualizationResources
A listing of useful PDMP-related websites, including: PDMP account registration, query, and data upload, PDMP statistics, Opioid Dashboard, PDMP user training, statutes/regulations, and opioid prescribing guidelines.
There has been growing concern among PDMP Administrators about the misinformation and misperception being disseminated at all levels regarding the workings and operations of these programs. PDMP Administrators around the country identified the need for the creation of an orientation package for new PDMP Administrators. This package could be used by new and less experienced PDMP Administrators as a guidance and reference tool. Revised November 2024
Analysis of prescription opioid overdose death statistics 2017-2021
This document summarizes the status of PDMPs, based on the results of the 2023 state assessment, related to operations, policies/procedures, technological capabilities, and authorized users.
This document provides a general overview of how prescription drug monitoring programs (PDMPs) operate.
The misuse of prescription drugs, especially opioids, has become a national crisis. The opioid epidemic creates a public health and safety concern that requires innovative solutions. One potential solution is the use of prescription drug monitoring programs (PDMPs). Research on the use of PDMPs provides evidence-based outcomes that can help inform decisions around public policy and provide insight into potential strategies to address the opioid crisis. PDMPs help detect fraud and provide valuable data that can be used to better understand the scope of the epidemic and the impact of various interventions. Research can also inform strategies for use of PDMPs to decrease opioid misuse and improve public health and safety.
This document summarizes the status of PDMPs, based on the results of the 2022 state assessment, related to operations, policies/procedures, technological capabilities, and authorized users.
This video podcast will provide an overview of Prescription Drug Monitoring Programs.
A copy of the transcipt is available here.
Recommendations for Best Practices on Dispenser Compliance and Data Integrity
View ReportReport — 08/29/2022
Complete and accurate prescription drug information is vitally important to support efforts to reduce the incidence of prescription drug misuse and diversion. Healthcare providers (prescribers and dispensers) have a vested interest in ensuring that quality data are transmitted to and available from prescription drug monitoring programs (PDMPs). This report examines promising practices for PDMPs in identifying the dispensers required to transmit prescription data, effective ways to monitor dispensers’ compliance, and how to provide dispensers with information about what data should be transmitted and how. It also examines where data errors may potentially occur and how PDMPs may identify the errors and processes to ensure timely corrective actions. This report details the areas where prescription data errors may occur, some procedures PDMPs employ to detect those errors, and steps to ensure that the erroneous data are corrected. In addition, it provides suggestions for prescribers, dispensers, and other stakeholders to prevent data errors from occurring.
Prescription Opioid Overdose Deaths: Analysis and Recommendations (rev. 2022)
View ReportReport — 02/16/2022
The PDMP Training and Technical Assistance Center (TTAC) compiled overdose death statistics from 1999 to 2020. PDMP TTAC analyzed the overdose statistics was to identify state efforts that were most effective in reducing the incidence of prescription opioid overdose deaths.
PDMP TTAC collaborated with representatives from the National Association of State Controlled Substances Authorities (NASCSA) PMP Committee to provide guidance surrounding veterinary prescription reporting to prescription drug monitoring programs (PDMPs). The “Recommended Best Practices for Veterinary Prescriptions” report examines the issues facing PDMPs as it relates to veterinarians and offers suggestions on how PDMPs may best address them. The suggestions are a consensus reached as result of the TTAC/NASCSA work group’s efforts and is intended to:
- show reporting issues of veterinary dispensing and prescribing,
- find issues in displaying such data in PDMP reports and queries, and
- offer recommended best practices on techniques and policies for PDMPs.
This document summarizes the status of PDMPs, based on the results of the 2021 state assessment, related to operations, policies/procedures, technological capabilities, and authorized users.
Patient privacy and confidentiality is extremely important for patient outcomes and it is also the law. Federal and state privacy laws, including those applicable to prescription drug monitoring programs (PDMPs), provide protection for patients who seek or obtain medical care, and there are heightened legal protections for the privacy substance use disorder (SUD) treatment information. 42 CFR Part 2 (known as “Part 2”) serves to protect patient records created by certain federally assisted programs for SUD treatment.
These regulations, “Confidentiality of Substance Use Disorder Patient Records,” were first promulgated in 1975 to address concerns about the potential use of SUD information against patients. 42 CFR Part 2 regulations are intended to ensure that a patient receiving SUD treatment from a 42 CFR Part 2 Program does not face adverse consequences.
Since 2010, the PDMP Training and Technical Assistance Center (TTAC), at the Institute for Intergovernmental Research (IIR), with support from the Bureau of Justice Assistance (BJA), has conducted seven (7) state assessments of prescription drug monitoring programs (PDMPs). The assessments have gathered data on PDMP statutes, regulations, policies, and procedures; tracked their changes over time; and identified program trends and candidate best practices. This document details the current status of PDMPs, based on the results of the 2020 state assessment, related to operations, policies/procedures, technological capabilities, authorized users, and PDMP reports.
This Technical Assistance Guide (TAG) is intended to (1) identify reporting issues of veterinary dispensing and prescribing, (2) identify issues in displaying such data in PDMP reports and queries, and (3) provide recommended best practices on techniques and policies for PDMPs.






